Viral pathogen detection with high accuracy and sensitivity is crucial in several settings. For example, in clinical practice, it enables timely diagnosis, effective treatment, and containment of infectious diseases. During the manufacturing process of biopharmaceuticals, it ensures the safety of the product, which otherwise could become ineffective or even harmful due to the presence of adventitious viruses.
GenomeScan utilizes highly sensitive Comprehensive Viral Research Panel developed by Twist Bioscience, capable of screening for >3,000 different viruses, including >15,000 viral strains, in a single reaction. Using this panel, we have completed a service validation and recently acquired Twist NGS ProLab certification.
Advantages of working with a Twist NGS ProLab
A Twist NGS ProLab offers you a reliable option for your lab to evaluate and incorporate new NGS workflows or enable scaling with larger or more complex sequencing studies. As a Twist NGS ProLab, GenomeScan is certified and fully supported to run Twist Target Enrichment and Library preparation solutions, giving you confidence in obtaining high-quality sequencing data so you can focus on your research.
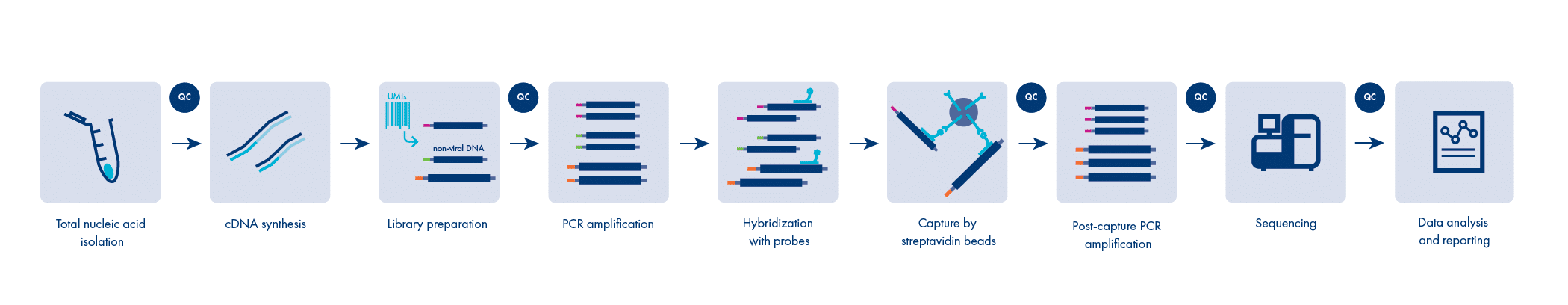
GenomeScan’s Comprehensive Viral Panel workflow
Our proprietary workflow can detect viruses of all nucleic acid genome types: single-stranded RNA, double-stranded RNA, single stranded DNA, and double-stranded DNA.
If you would like to learn more about our solutions to detect extraneous agents, please visit our relevant webpage.
Clinical and biopharmaceutical applications
NGS offers short turnaround and high specificity as well as sensitivity, thus utilizing Comprehensive Viral Panel provides advantages over traditional methods both in clinical and manufacturing settings.
To determine the sensitivity and specificity of our viral pathogen detection workflow, we performed a thorough experimental validation using different spike-in controls including ssRNA, dsRNA and dsDNA viruses. Below, you can download our technical leaflets for clinical molecular diagnostics or biomanufacturing applications that outline this method and its outcome.