Verify the genetic identity and stability of your producer cell line
Regulatory bodies worldwide require a thorough genetic characterization of producer cell lines across the entire manufacturing process. Next Generation Sequencing (NGS), cutting-edge technology that guarantees in-depth evaluation of the identity and stability of your model systems, is the key. Whether it is Master Cell Bank (MCB), Working Cell Bank (WCB) or End of Production Cell Bank (EOPC), our range of NGS-based genetic characterization services are now within your reach.

Discuss your project with us
Our solutions
Vector quality control
An extensive genetic characterization of cell line is important at all stages of drug development: from the initial transformation to compound production. GenomeScan provides NGS-based tools to accurately characterize the expression constructs that are used in the production of recombinant DNA. Whether you are verifying the sequence of your plasmids or you are seeking to confirm the identity and serotype of your viral vector, GenomeScan will match appropriate solution tailored to your specific needs.
We routinely sequence:
- PCR products
- plasmids e.g. used to generate cell lines, viral vectors
- viral vectors e.g. frequently used in manufacturing of vaccines and gene therapies (adeno-associated viruses, adenoviruses, lentiviruses, retroviruses)
Cell line characterization
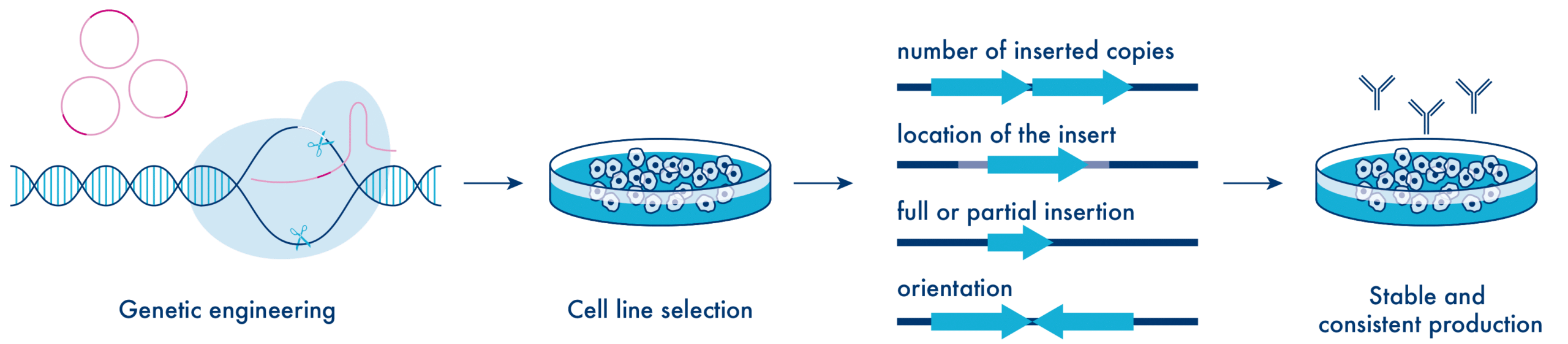
Generation of producer cell lines is not trivial. The transfected gene insert may dramatically affect clonal stability. It is critical to evaluate the insert location (risk of gene disruption at insert site) and to determine the number of inserted copies into the genome. Furthermore, it must be ascertained whether a full or partial insertion of the target sequence has occurred and whether the orientation of the integrated construct(s) is correct.
In any facility utilizing cell lines for development and manufacturing purposes, an extensive genetic characterization of the models used is paramount. Whole Genome Sequencing (WGS) offers the most accurate and extensive characterization of your producer cell line, with an unmatched resolution at the base pair level. To achieve the best results, we recommend using a combination of short and long-read sequencing technologies.
Cell line authentication
Continuous expansion of cell cultures leads to genetic alterations that often emerge unnoticed. Single nucleotide polymorphisms (SNPs), insertions/deletions (indels) and copy number variations (CNVs), frequently impact the stability of the cell line and its production capabilities. In this context, the classification of the functional consequences of the aforementioned alterations is particularly relevant: a stop-inducing SNP or a synonymous mutation will have very different biological consequences.
Routine monitoring of producer cell lines with NGS is now easily attainable, fast and cost-effective. To ensure stable and consistent production, we recommend authenticating your cell line banks regularly. Our cell line authentication service compares the genetic information of your Working Cell Bank (WCB) or End of Production Cell Bank (EOPC) with your Master Cell Bank (MCB) reference.

Key benefits
Our NGS-based solutions for genetic characterization:
- are GMP compliant
- are compatible with a wide range of materials
- aid stable and consistent production
- accelerate your manufacturing processes
- reduce costs

Service specifications
We have summarized key information about our genetic characterization service into a service specification sheet.
What our customers say
“GenomeScan performed cell line authentication (CLA) sequencing services that exactly met my needs. The job was performed very quickly and the bioinformatic processing was tailored to my needs. Especially the communication was clear and concise, all agreements were honoured.”
Scientist
University Medical Center, The Netherlands
“Had the pleasure of interacting with Magda and Gerben for a WGS project and was highly impressed by the data quality, professionalism and competence of the personnel at Genome Scan. The turnaround times for the data generated and the subsequent analyses was very quick. Look forward to working with them on other projects.”
Associate Director, Process Development, Vinay Vyas
Frederick National Laboratory for Cancer Research, Operated by Leidos Biomed
Let's get the conversation started for your next NGS project
Let us help you to design and implement a comprehensive genetic characterization program, tailored to your specific requirements. Please fill out the form below or email us directly at geneticQC@genomescan.nl and we will get in touch with you to discuss your requirements.

