Profile your clinical research cohort or match a patient with available targeted therapies according to their tumour’s genetic profile.

Service details
Pan cancer panels can be useful for in-vitro diagnostics (IVD) and as a clinical research solution. Clinically available targeted therapies are growing in number, and even more are currently being investigated or evaluated in clinical trials. These include drugs targeting specific mutated genes or activated pathways, and immunotherapy targeting tumour-specific neoantigens. And with that, it has become evident that comprehensive tumour profiling is warranted for clinical cohort analysis and clinical profiling. Simultaneous assessment of all major genetic biomarkers and drug targets in a single assay is the solution for the often-limited amount of DNA that is available for genetic analyses.
Where a comprehensive cancer panel’s MSI and TMB analysis can serve as a biomarker for response to immunotherapy, the pan-cancer content is aligned with key guidelines and clinical trials to assess biomarkers and drug targets important for targeted therapies.
Presently, we offer Illumina’s TrueSight Oncology Comprehensive (EU) DNA panel (Kroese et al.) – in short TSO500 DNA – as full assay or ready-to-run option. Aligned combination with RNA profiling is also a possibility. Send in your samples, isolated DNA, or prepared libraries, and we provide you with the analysed data generated using the dedicated variant calling software. For pricing and turnaround times please contact us.
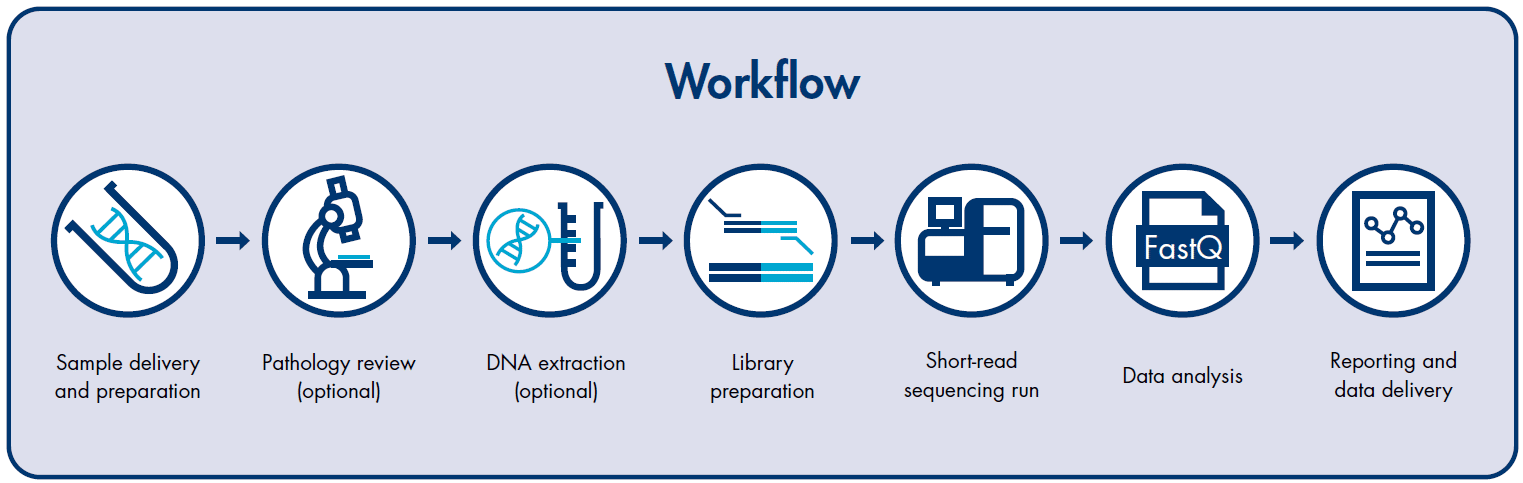
Comprehensive Cancer Panel workflow
The workflow of comprehensive cancer panel analysis involves several key steps. Initially, tumor samples, obtained through biopsies or liquid biopsies, undergo DNA or RNA extraction. The extracted genetic material is then prepared as libraries using specialized protocols tailored to the comprehensive panel design.
These libraries are subjected to NGS using cutting-edge sequencing platforms, such as Illumina, generating vast amounts of sequencing data. Advanced bioinformatics pipelines are employed to process and analyze the data, identifying genetic alterations within the targeted genomic regions. Annotation databases and bioinformatics tools aid in interpreting the detected alterations, providing crucial insights into potential driver mutations, therapeutic targets, and prognostic markers
Key benefits
- Assessment of all major genetic biomarkers and drug targets in a single assay.
- Clinically relevant turnaround times.
- Performed under ISO/IEC 17025 accreditation.
- Data analysis provided using dedicated Illumina variant calling software.
We have summarized key information about Comprehensive Cancer Panel solution into a service specification sheet.
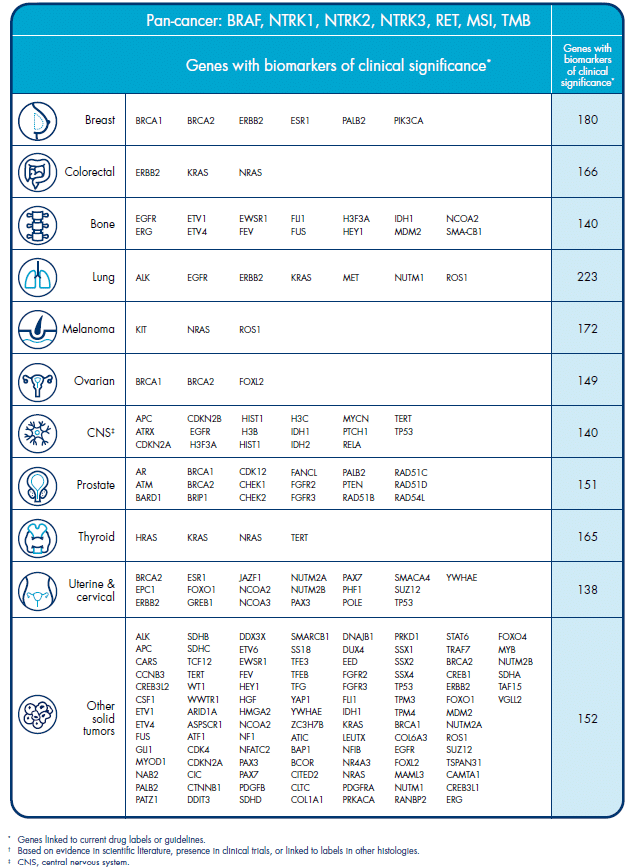
Here, you can download a list of genes we cover with biomarkers of clinical significance
Let's get the conversation started for your next NGS project
Please either fill in below or email us directly at oncology@genomescan.nl
and we will get in touch with you to discuss your requirements

