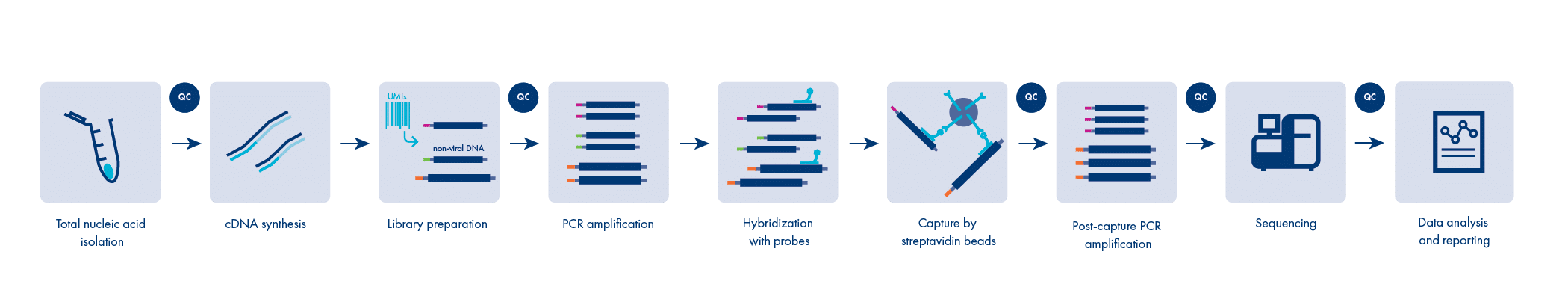
Identify and quantify adventitious agents present in your sample
The safety of vaccines, monoclonal antibodies, cell and gene therapy products and other biologically-derived products is strictly regulated and required for a product release. Specific in-vivo and in-vitro assays are utilized to assess the purity of biologics. However, performing numerous tests on each product requiring clearance is laborious, lengthy and expensive.
Implementing Next Generation Sequencing (NGS) within your safety testing programme offers unparalleled specificity, reliability, accuracy and speed. NGS is a unique, unbiased approach that detects both known and unknown contaminants. It is unmatched by conventionally applied methods. Notably, detection of adventitious agents with NGS is encouraged by the World Health Organization (WHO), European Pharmacopeia (Ph. Eur.) and U.S. Food and Drug Administration (FDA).
With our proprietary sample preparation, combined with tailored sequencing and bioinformatics solutions, we enhance your quality control throughout the entire manufacturing process. Our assays are compatible with a wide range of products (raw materials, ancillary reagents, virus seeds, cell substrates, cell banks, pre-production cells, pre-filtered harvest, post-production cells, control cells, post-filtered harvest and final product), and have the capacity to identify a contamination of any origin, whether it is viral, bacterial or fungal.

Discuss your project with us
Our solutions
Comprehensive Viral Panel
A hybridization-based target enrichment solution that is capable of detecting > 3,000 different viruses, including > 15,000 viral strains in a single reaction
Viral Metagenomics
Detect and characterize the diversity and abundance of viruses, including those that may be previously unknown, with an agnostic approach

Key benefits
Our NGS-based solutions for detection of adventitious agents:
- replace multiple tests with a single, comprehensive assay
- de-risk and accelerate your manufacturing processes
- improve sensitivity and accuracy of your biosafety testing
- reduce costs

Service specifications
We have summarized key information about our detection of adventitious agents service into a service specification sheet.
Let's get the conversation started for your next NGS project
Let us help you to design and implement a comprehensive detection of adventitious agents programme tailored to your specific requirements. Please fill out the form below or email us directly at geneticQC@genomescan.nl and we will get in touch with you to discuss your requirements.