Genetic QC
Accelerate and de-risk your biomanufacturing with Next Generation Sequencing.
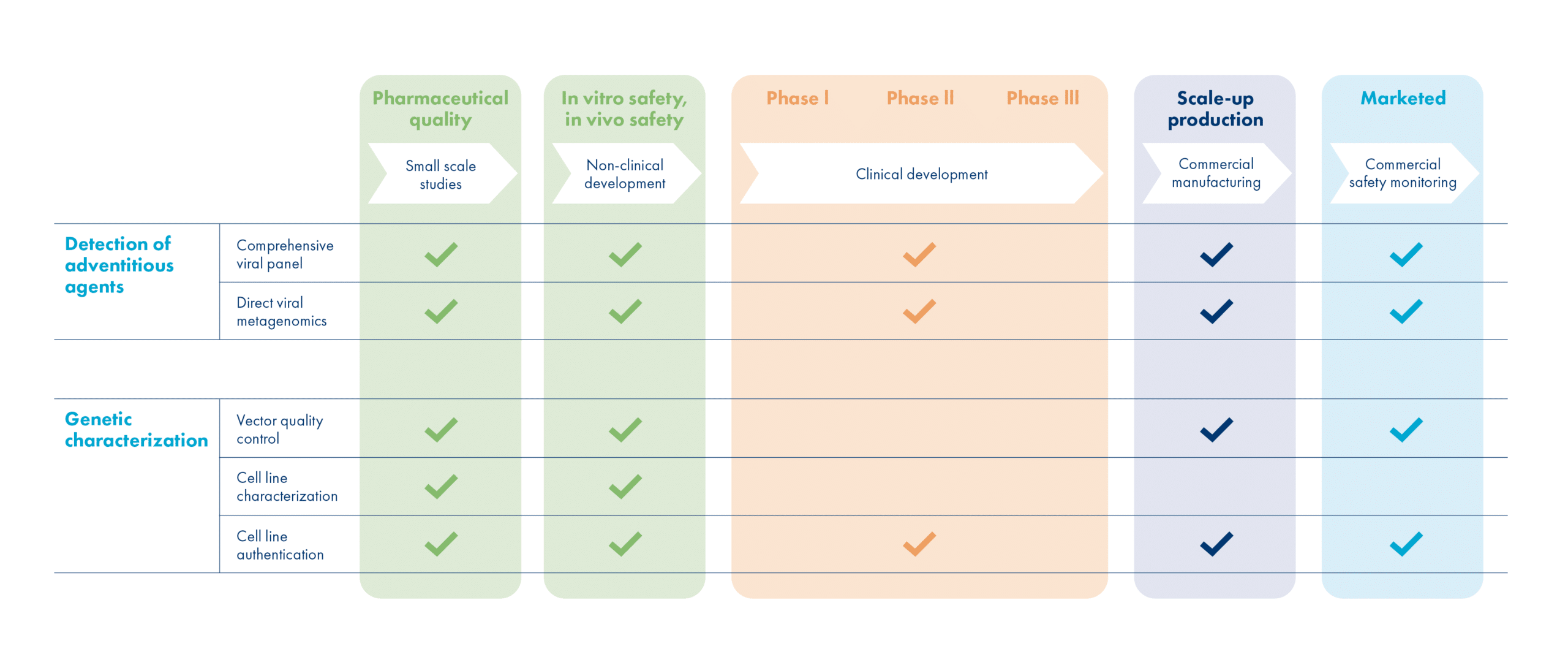
GenomeScan offers a comprehensive array of test methods to ensure that your biopharmaceutical products are free from (genetic) contaminants. Our NGS-based solutions are a perfect match for various applications and provide you multiple advantages.
“GenomeScan performed cell line authentication (CLA) sequencing services that exactly met my needs. The job was performed very quickly and the bioinformatic processing was tailored to my needs. Especially the communication was clear and concise, all agreements were honoured.”
Scientist
University Medical Center, The Netherlands
“Had the pleasure of interacting with Magda and Gerben for a WGS project and was highly impressed by the data quality, professionalism and competence of the personnel at Genome Scan. The turnaround times for the data generated and the subsequent analyses was very quick. Look forward to working with them on other projects.”
Associate Director, Process Development, Vinay Vyas
Frederick National Laboratory for Cancer Research, Operated by Leidos Biomed
Please fill out the form below or email us directly at geneticQC@genomescan.nl and we will get in touch with you to discuss your requirements